Geol: 2081: Mineralogy
(c) copyright B. Dutrow 2006
"Minerals are the basic stuff of the Earth, and their
study will always remain at the core of the Earth Sciences."
-
Frank Hawthorne, 1993

|
Minerals: We walk on them, we wear them, we wash our
clothes with them, we may even brush our teeth with them. They bring electricity
into our lives and provide the building materials for our homes and schools.
Without minerals, we would not have an Earth!
|
Banded Iron Formation - Australia
|
|
Goals of the course
To understand what these
materials are, that are an integral portion of our life:
|
As citizen's of the Earth,
and as students of geology.

|
To provide a solid foundation
in conceptual aspects of mineralogy:
|
What minerals are, how they
form, how they are analyzed and described, how they behave as a function
of P-T-X, how they are classified, identified, and utilized.
|
To understand their utility
in the study of our Earth:
|
Because these materials are
the products of complex Earth and Planetary processses that occur over a
wide range of temperatures and pressures, minerals can tell us conditions
of formation and subsequent evolution of the continents and ocean basins.

Pallasite meteorite, photo courtsey of the Smithsonian
Institution
|
To provide linkages to
other areas of geology:
|
petrology, geochemistry,
geophysics, structural geology, meteoritics, environmental geology, geomicrobiology,
economic geology
Hawthorne, 1993

Recent and fossilized pinecones
|
To connect with
other fields of study:
|
inorganic chemistry, material
science, gemology, soil science, biology, nanoscience, art, archeology
Uxelite, a mineral that behaves as a fiber optic
|
To understand their central
role in our standard of living,
|

|
Have fun learning how the Earth
works!
|
|
We will apply principles learned from each portion
of the course as we proceed......
Lecture material concentrates
on concepts and principles
Lab exercises put these
principles to use where we descibe and identify hand specimens
Mineralogy & MINERALS

|
Mineralogy, or Mineral
Science, is the study of minerals.
This encompasses:
- crystallography (where we'll start)
- how chemical elements make minerals
- crystal chemistry
- relates chemical composition, internal structure, and
- physical properties
- identification
- classification
- geologic occurrence
- how and where they form
|
| Staurolite, Russia |
|
*
We are all familiar with minerals
We are all familiar with Minerals
|
|

diamond in matrix
|
Gems
|
|
|
Beaches
|
White, Black, Green, Pink
|
Household items
|
Wiring
|
Mud after a rainstorm
|
"Gumbo"
|
|
|
Minerals are the building blocks of
the EARTH!
Basic units from which rocks
and everything else in the Earth are comprised
Metamorphic rocks, Campolungo, Switzerland
|

|
Minerals as Key Components
to Geologists
|
|
- Rocks are classified based on their Mineralogy
- granites = plagioclase, orthoclase,
quartz
- basalt = plagioclase, olivine, pyroxene
- eclogite = garnet + pyroxene
|

|
|
Eclogite, Switzerland
|
- Minerals provide
clues/answers to a rock's formation
|
Different minerals form in
different geologic environments.
- they may tell of pressure, temperature
conditions,
fluid or melt composition, oxygen fugacity, etc.
|
|
|
Minerals provide important natural resources (Economic
Geology)
Essential to our standard
of living
|
- from our ancestors (fire,
arrowheads) to today's superconductors
we depend on terrestrial raw materials for everything that makes
our life above subsistence level
|

|
e.g. metals (Cu, Au, Ag), diamonds,
or store
important reserves: oil, gas
Native Copper in Veins - AZ
|
Each year, every American requires 40,000 lbs
of new minerals!
|
Can the world sustain this
rate of consumption?
|

|
Massive Sulfides, Czech Republic
|
They provide solutions to environmental
problems
|
e.g. Absorbants - Zeolites,
Clay minerals (kitty liter)
- Retard migration of toxic chemicals
|
They cause some environmental
problems
|
acid mine drainage, asbestos,
erionite
|
We'll develop a feel for the diversity of minerals
and their behavior in a wide variety of environments
One of the unifing themes of mineralolgy is that
of crystal chemistry; the elucidation of the relationship between chemical composition, internal structure and physical
properties.
WHAT IS A MINERAL?
Naturally occurring
|
formed by natural processes
synthetic = lab made
|
Solid
|
excludes liquids and gases
|
Crystalline
|
highly ordered atomic arrangement,
an internal structural framework of atoms (ions) arranged in a regular,
repeating, geometric pattern (periodic)
solids that lack an ordered atomic arrangement are amorphous
|
definite chemical composition
|
definite but not fixed, within
limits (or range) of compositions and can be expressed as a chemical formula:
e.g. FeS
|
formed by inorganic processes,
usually
|
it is increasingly recognized
that minerals may be produced organically e.g. magnetite in bird brains,
aragonite in shells = biomineralization
|

plagioclase feldspar, labradorite, Madagascar
|
There are ~ 4200 known mineral species.
- About 100 are common (we will learn these)
- a few dozen are considered abundant.
This is minor compared to the biological world;
there are more than 10,000 species of inchworms
alone!
|
Mineraloids

|
mineral-like but lack all
the qualifications (opals)
|
Naming of minerals
|
after properties, place, people
|
| Minerals |
|
Not minerals |
| |
|
|
| quartz - SiO2 |
|
opal |
| ice |
|
CO2 |
| snow |
|
granite |
| biotite |
|
volcanic glass |
| diamond |
|
oil |
| serpentine |
|
amber |
| |
|
|
WHAT IS A ROCK?
| solid |
|
cohesive
|
|
Aggregrate of grains
|
|
made of one or more minerals
|
|
Granite Slab - Sardinia |

|
|
The study of rocks is PETROLOGY.
|

|
Epidote and Quartz, Madagascar
|
|
|
|
|
-
Examples
| Rock |
|
Not a rock |
| |
|
|
| quartzite |
|
quartz |
| |
|
|
| granite |
|
sand, soil |
HISTORY of MINERALOGY
Began with mineral's Uses
|
Minerals and rock were selected
and used for certain purposes
long before humans even devised a written language.
|
|
The prehistory of humanity
is dated by the materials with which our predecessors made their tools.
|
|
One of our first scientific
acts was to distinguish between different rocks and minerals and use them as tools according to their properties.
|
"Stone and the Stone
Age"
|
|
|
Our early ancestors were vegetarians,
when their ancestors became omnivorous, the use of tools was essential.
|
|
Realized suitibility of certain
materials,
e.g. obsidian and flint became widely used.
|
|
Fire was started
by striking pyrite with flint.
Naturally-occurring poisons such as arsenic
were known and used.
|
|
Toward the end of the Neolithic,
ca. 40 different rocks and minerals were in use.
|
"Metals and the Bronze Age"
|
|
|
Naturally occurring native metals
were greatly sought after by Stone age humanity
for their curiosity and ornamental value rather than for tool making.
|
|
Discovered that heating metals greatly promotes
the ease with which they can be worked and extracted.
e.g. the origin of the discovery of bronze; early
bronze was a mixture of Cu and As.
|
|
Metals have been used for at
least 4000 yrs; prehistoric man was also known to mine and smelt metallic minerals to produce Au,
Ag, Fe, Cu, Pb and bronze.
e.g. Hg was in use in Egypt as early as the
15th-16th C BC and
the old Testament of the Bible refers
to the use of Au, Ag, Cu, Sn, Pb and Fe.
|
"Iron Age"
|
|
|
About 1400 B.C.
|
|
Fe is hard, durable and abundant
|
|
Throughout the Stone, Bronze
and Iron ages, humanity also began to use an increasing number of
minerals unrelated to the manufacture of tools.
|
ART
|
Fascination with ART seems an
intrinsic property of the human psyche.
Cave art is our earliest record.
|
|
Colored pigments became common;
red and black pigments (hematite and pyrolusite, Mn oxides)
were used for cave painting for over 5000 yrs.
|
|
Other minerals (jade, amethyst,
garnet) were used for ornamentation or the diverse
hard minerals and rocks
used for tools and weapons (jade, flint,
obsidian).
|
|
|
|
|
-
Records

* Indian, 1100 B.C.
* First written work on minerals
is by Greek philosopher Theophrastus
(372-287
B.C.) and Pliny 300 yrs later.
* Emergence of mineralogy as a science
is credited to German physican
Georgius
Agricola with the publication De Re Metallica in 1556.
* 1669 Steno and his law.
Interfacial angles of quartz xls are constant, no
matter what the size and shape of the crystal is.
This discovery lead to the science of crystallography.
He was beatified in 1988 by the pope
* followed by the physics of minerals
-early 19th
century, rapid advances with the
development of instruments which could measure
precisely angles between xl faces.
* the chemistry on minerals.
-
1854 Dana introduced the chemical classification used today.
e.g. bk. After J.D. Dana...
* 20th century,
- 1914
Braggs solved the first crystal structure.
* Now rapid advances in instrumentation
and techniques for measuring and imaging
exact positions of atoms in a crystal.
Linus Pauling, the Nature of the Chemical Bond
CRYSTALLOGRAPHY
1. CRYSTALLINE - characteristic of a mineral;
long range 3D internal structural order
2. CRYSTAL - when external form is bounded by
geometric forms
- external
form is function of growth environment and may not reflect internal order.
We use term
in broader sense to indicate ordered 3D structure.

|
| Staurolite, Urals, Russia |
3. Crystal GROWTH
- grow from
nucleus by accretion from soln, melt or vapor
- grow from
outside cf. plants
-
amount of space available for growth determines shape
4. MORPHOLOGY - external form of mineral
(a) euhedral = well formed faces

|
| Pyrite, Spain |
(b) subhedral = partial xl faces
(c) anhedral = no xl faces
(d) microcrystalline = xls observable
with microscope
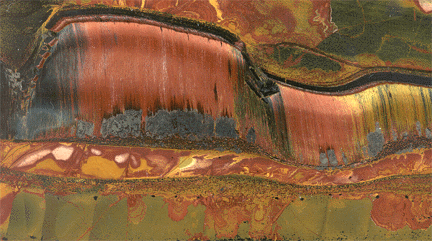
|
| Tiger's Eye, Australia (SiO2 replaced Amphibole) |
(e )cryptocrystalline = xl resolved
with x-rays
(f) amorphous = no long range order

|
| Opal, Australia |
5. CRYSTALLOGRAPHY - study of crystalline materials
(Steno),
crystal
symmetry, orientation of atoms in xl.
(a) direct imaging of internal arrangement
e.g. TEM
(b) relationship between xl faces
- symmetry
Two methods to describe positions of atoms in xline
matter:
(a)Translational - spatial relationships
(b)Rotational - relationships between
faces
B. dutrow
2006-08-27